Our Responsibility ⟩ Global Medical Affairs
Amicus Therapeutics supports programs and activities that foster excellence in patient care and provide valuable scientific, medical, and educational information to the medical and scientific communities. Grant funding for an accredited Continuing Medical Education (CME) program (i.e., ACCME, AMA, AAFP, ADA, or AOA) or continuing education for other allied health professions (e.g., nurses, pharmacists), from U.S.-based continuing education entities (e.g., academic institutions, education program vendors) will be considered. Since available grant funding is limited, selection priority is given to exceptional high quality, impactful educational activities which serve to close gaps in health care provider (HCP) knowledge, skills, and professional performance used to provide services to patients, the public, or the profession. Amicus Therapeutics will not influence the content nor have control over those educational programs funded through Global Medical Affairs as independent medical education grants. An assessment plan of participant outcomes is a requirement for funding.
Note: Grant requests should be submitted on the Amicus IME portal by the accredited provider.
Amicus funds non-accredited activities aimed at educating health care professionals, researchers and scientists. Non-accredited continuing medical education (CME) grants are held to the same standards of independence as accredited CME grants including requirements related to content, influence, faculty selection, educational methods, materials, venues and attendees. Examples of non-certified CE educational programs that Amicus Therapeutics can potentially support include, but are not limited to, the following:
Please note that Amicus Therapeutics will not provide educational grant funding for the following:
Amicus will consider funding certified educational grants for programs that address the following rare disease states:
IME funding requests are accepted for review on an ongoing basis and can be submitted at any time. Requests must be received no later than 90 days before the activity start date. Under no circumstances will IME funding requests be considered for programs that have taken place or started in the past.
Amicus Therapeutics, Inc., reserves the right to modify, revise or delete therapeutic areas of interest, and other terms and conditions of its IME funding program, at any time and without notice.
Requests for Education (RFE) may be published by Amicus periodically in therapeutic or disease state areas of interest based on educational gaps that are identified. If your organization is interested in receiving Amicus RFEs, please send us an email at GMA_IME@amicusrx.com.
Submit an application through our secure online portal, where you can track the status of your request and communicate with Amicus. The application captures the following information:
If you are applying for an IME Grant for the first time, Register Here. To continue an online application, or to check on the status of an IME Grant application, Login Here. If you have additional questions, email us at GMA_IME@amicusrx.com.
The online portal allows you to track the progression of your grant request, and communicate with Amicus:
MED-US-NN-2400003
IMPORTANT: Amicus has implemented application windows for unsolicited requests. The window is currently closed. Please check back periodically for updates.
At Amicus Therapeutics (Amicus), we are committed to providing rigorous, groundbreaking science with real impact for people living with rare metabolic diseases. Investigator Initiated Studies (IIS) play an important role in advancing science by addressing the unmet research needs or areas of interest surrounding Amicus’ medical and scientific interests.
Amicus has a competitive research grant review process with specific requirements that must be met in order for your application to be evaluated. IIS proposal applications are reviewed based on scientific merit, alignment with areas of interest, budget availability, and other considerations.
Amicus provides support for independent research on topics that are scientifically sound and within the published areas of research interest. It is expected that results from IIS projects are communicated in scientific forums and/or published in peer-reviewed journals.
We welcome the scientific and academic research communities to submit high-quality IIS proposals through our research application portal. New applications are accepted in cycles during the timeframes designated below.
The IIS grant type supports research which may or may not include the study of an Amicus product. Clinical studies, observational studies, such as epidemiology studies, outcomes studies, non-clinical studies (i.e., laboratory or animal studies), as well as other types of independent research will be considered:
The types of support available include:
Amicus will consider support of independent studies meeting the following criteria:
Fabry Disease
Pompe Disease
*Amicus reserves the right to modify, revise or delete the Areas of Interest and other terms and conditions of its IIS program at any time without notice.
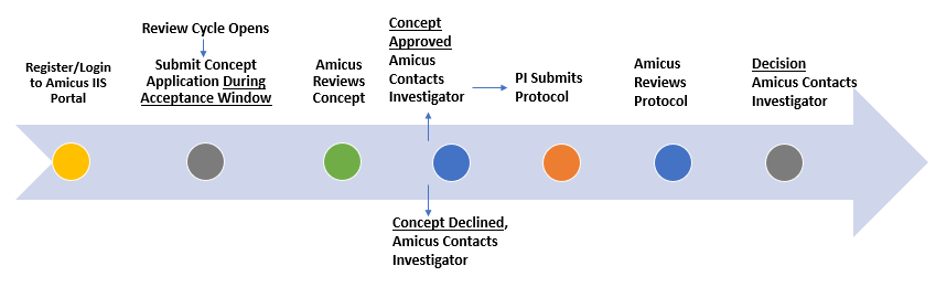
The IIS review process begins with submission of a study concept. The concept document and a copy of your signed and dated CV must be uploaded onto the IIS portal.
Amicus strives to provide a response to your concept application within 30 days of the scheduled review date.
Acceptance or approval of the concept does not guarantee support of a study.
If your IIS concept application has been approved, you will be notified of the decision and can begin drafting the study protocol. To facilitate this process and ensure all necessary information is reflected in the protocol, we provide a template that can be found on the Welcome Page of the IIS portal.
Submit your research protocol, budget (template provided), and other supporting documents.
New Users REGISTER on IIS Portal – to begin the Concept Application submission process.
Returning Users GO to IIS PORTAL – to track the status of your application.
Amicus strives to provide a response to your protocol application within 30 days of the scheduled review date.
Note: A proposal requesting Amicus support (e.g., funding and/or drug supply) is not a guarantee of acceptance or approval of that proposal. Amicus support will only be provided upon the execution of an agreement.
Thank you for your interest in the Amicus Therapeutics, Global Medical Affairs Investigator-Initiated Studies. Please email the Investigator Initiated Studies Administrator at Investigator-InitiatedProgram@amicusrx.com with additional questions.
MED-US-NN-2400003
For more information:
Medical Education Program Resources:
Investigator-Initiated Study Resources:
* (Provided as guide to help with development of robust protocol)