Responsibility ❯ Global Medical Affairs
↓ Independent Medical Education ↓ Investigator-Initiated Studies
Independent Medical Education Grants
Accredited Medical Education Grants (U.S.)
Amicus Therapeutics supports programs and activities that foster excellence in patient care and provide valuable scientific, medical, and educational information to the medical and scientific communities. Grant funding for an accredited Continuing Medical Education (CME) program (i.e., ACCME, AMA, AAFP, ADA, or AOA) or continuing education for other allied health professions (e.g., nurses, pharmacists), from U.S.-based continuing education entities (e.g., academic institutions, education program vendors) will be considered. Since available grant funding is limited, selection priority is given to exceptional high quality, impactful educational activities (Moore’s Outcome Level 4 or higher) which serve to close gaps in health care provider (HCP) knowledge, skills, and professional performance used to provide services to patients, the public, or the profession. Amicus Therapeutics will not influence the content nor have control over those educational programs funded through Global Medical Affairs as independent medical education grants. An assessment plan of participant outcomes is a requirement for funding.
Non-Accredited Medical Education Grants
Amicus Therapeutics applies the same standards of independence to non-accredited continuing education (CE) grants with respect to content, influence, faculty selection, educational methods, materials, venues and attendees as it does to accredited CE grants. Examples of non-certified CE educational programs that Amicus Therapeutics can potentially support include, but are not limited to, the following:
- Lectures or other educational activities
- Managed care-sponsored educational activities
- Grants to support healthcare-related public policy initiatives
- Disease state and treatment education and awareness
Overview
- Amicus Therapeutics is compliant with federal and state laws, as well as guidelines that govern such activities. Amicus Therapeutics commercial staff, including field staff, are not involved in decisions to fund IME programs.
- Responsibility and control over the selection, content, faculty, educational methods, materials, and venue for an independent medical education event belongs solely to the organizers of the event in accordance with the accredited provider’s guidelines.
- Amicus Therapeutics will not provide any advice or guidance to the accredited independent medical education provider (including, if requested) regarding program content or faculty for any Independent Medical Education program.
- Amicus Therapeutics requires that a fully-executed Letter of Agreement (LOA), in the form provided by Amicus Therapeutics, be signed by the requesting Continuing Education Provider and Amicus Therapeutics Global Medical Affairs Independent Medical Education prior to the commencement of the Continuing Education program. Continuing education programs that have commenced in the past will not be approved nor funded by Amicus Therapeutics.
- For those funding requests approved by Amicus Therapeutics, the IME funding recipient is required to confirm the completion of the activity and to provide documentation of how the funds were used within 90 days after the completion of the independent medical education activity. Reconciliation documentation should be uploaded to the grant application through the Amicus IME Grant Portal.
- Funds provided by Amicus Therapeutics in support of an IME program and not spent in the execution of the program must be returned to Amicus Therapeutics.
Please note that Amicus Therapeutics will not provide educational grant funding for the following:
- Individuals
- Requests for payments to Individuals
- Fellowships
- Overhead Expenses
- General Operating Expenses
- Entertainment Expenses
- Awards / Travel Awards
- Costs of travel, lodging, or other personal expenses of non-faculty individuals attending IME, either directly to the individuals participating in the event or indirectly to the event’s provider.
- Funding used to compensate participants for time spent at the IME event.
- Debarred institutions or groups, including organizations or their employees on probation with the ACCME or other organizations, or on the OIG exclusion list; as well as faculty debarred from a federal healthcare program or on the OIG exclusion list.
Therapeutic/Disease State Areas of Interest
Amicus will consider funding certified educational grants for programs that address the following rare disease states:
- Fabry disease
- Pompe disease
IME funding requests are accepted for review on an ongoing basis and can be submitted at any time. Requests must be received no later than 90 days before the activity start date. Under no circumstances will IME funding requests be considered for programs that have taken place or started in the past.
Amicus Therapeutics, Inc., reserves the right to modify, revise or delete therapeutic areas of interest, and other terms and conditions of its IME funding program, at any time and without notice.
Request for Education
Requests for Education (RFE) may be published by Amicus periodically in therapeutic or disease state areas of interest based on educational gaps that are identified. If your organization is interested in receiving Amicus RFEs, please send us an email at GMA_IME@amicusrx.com.
Grant Submission Process
Submit an application through our secure online portal, where you can track the status of your request and communicate with Amicus. The application captures the following information:
- Unmet needs or educational gap assessment
- Educational objectives
- Program details:
- Program title, date(s), and location(s)
- Description of the proposed activity
- Identification of the target audience and projected number of attendees.
- Name of accrediting body and accredited provider, including type and number of medical education credits offered for the proposed activity (program must be accredited).
- If applicable, identification of co-requesting organization(s), medical education partner(s), and/or logistical partner, including contact information for these organizations.
- Whether or not the requestor is seeking co-funding support from other companies or entities.
- Amount of funding requested from Amicus Therapeutics
- Signed and dated by authorized representative
- Proposed agenda (draft acceptable)
- Proposed program outcomes assessment (e.g., pre- and post-test scores for the participants, participant survey with respect to the quality and / or utility of the educational program).
- Full, itemized program budget (must use Amicus budget template)
- If applicable, a copy of the organization’s non-profit determination letter from the IRS.
- Current IRS W-9 form or equivalent foreign documentation (must be signed and dated within the last 12 months).
If you are applying for the first time, Register Here. To continue an online application, or to check on the status of an application, Login Here. If you have additional questions, email us at GMA_IME@amicusrx.com.
Non-accredited Medical Education
Amicus funds unaccredited activities aimed at educating health care professionals, researchers, scientists, or patients, such as health-related public conferences, symposia, or community awareness campaigns where the program includes topics relating to disease management, therapy options and treatment.
If you are applying for the first time, Register Here. To continue an online application, or to check on the status of an application, Login Here. If you have additional questions, email us at GMA_IME@amicusrx.com.
Notification of Decision
The online portal allows you to track the progression of your grant request, and communicate with Amicus:
- A confirmation email with an identification number is sent to the Requestor immediately following a submission
- Requests for additional information are managed directly through the online portal
- Once the review process is complete, an email notification with the decision is sent to the Requester
- If grant request is approved, a Letter of Agreement will be sent to the Requester. Amicus Therapeutics requires that its form of agreement is fully executed prior to the start date of the program, content development or activity
Reconciliation
- A completed reconciled budget form must be uploaded onto the approved grant via the online portal within ninety (90) days of the program end date
- If the completed reconciled budget, or any other requested documentation are not received within the specified timeframes, the recipient will be precluded from receiving additional education funding
- All unused funds must be returned to Amicus
- The provision of outcome data (e.g., number of participants, participant areas of specialty, pre- and post-test scores, participant surveys) that measures effectiveness, value, benefit, or impact of a completed (live and/or enduring) IME program or event is required as a condition of funding
MED-AS-NN-2000028
Investigator-Initiated Studies
IMPORTANT: Amicus has implemented application windows for unsolicited requests. See the schedule below.
At Amicus Therapeutics (Amicus), we are committed to providing rigorous, groundbreaking science with real impact for people living with rare metabolic diseases. Investigator Initiated Studies (IIS) play an important role in advancing science by addressing the unmet research needs or areas of interest surrounding Amicus’ medical and scientific interests.
Amicus has a competitive research grant review process with specific requirements that must be met in order for your application to be evaluated. IIS proposal applications are reviewed based on scientific merit, alignment with areas of interest, budget availability, and other considerations.
Amicus provides support for independent research on topics that are scientifically sound and within the published areas of research interest. It is expected that results from IIS projects are communicated in scientific forums and/or published in peer-reviewed journals.
We welcome the scientific and academic research communities to submit high-quality IIS proposals through our research application portal. New applications are accepted in cycles during the timeframes designated below.
Suitable Types of IIS Proposals:
The IIS grant type supports research which may or may not include the study of an Amicus product. Clinical studies, observational studies, such as epidemiology studies, outcomes studies, non-clinical studies (i.e., laboratory or animal studies), as well as other types of independent research will be considered:
The types of support available include:
- Fabry disease- Funding and drug
- Pompe disease- Funding only
2025 IIS Application Windows and Review Cycle:
Amicus will accept applications for IIS support during the following windows. Additional application windows may open throughout the year.
| Topic | Requesting Organization Location | Concept Submission Window | |
|---|---|---|---|
| Open | Close | ||
| Fabry Disease | US and Ex-US | April 16, 2025 | May 21, 2025 |
| Pompe Disease | – | – | – |
Application Criteria
Amicus will consider support of independent studies meeting the following criteria:
- The study type is clinical, observational, epidemiological, an outcomes study or non-clinical (i.e., laboratory or animal) studies.
- If approved, the requesting organization will be the contracting organization to which the grant will be paid.
- Grants are not provided to individuals, individually owned private physician practices, or informal groups which are not legal entities.
- To submit your request to Amicus, you must answer the questions in the online application. Paper applications will not be accepted.
- All application fields must be completed in English and any uploaded documents must be in English.
2025 IIS Areas of Interest*:
Fabry Disease
- Diagnosis
- Shortening the pathway to diagnosis in undiagnosed/misdiagnosed symptomatic patients across diverse groups (gender race ethnicity)
- Real World Evidence
- Describe migalastat real world Fabry cohorts that explore the relationship between clinical parameters and disease outcomes, including treatment pathways and QoL measures
- Underserved populations and health inequities
- Describe the natural/treated history and disease burden of underserved populations in Fabry disease (e.g. females) to inform clinical management
- Tissue distribution and efficacy of treatment
- Explore the role of tissue distribution and penetration and the relationship to treatment response and outcomes in Fabry disease patients with various manifestations (e.g., neurologic, cerebrovascular, cardiac)
- Timely Clinical Management
- Technological advances and scientific discovery to inform appropriate timely management strategies
- Exploration of the benefits of timely management on clinical outcomes
Pompe Disease
- Real World Evidence
- Describe real world cohorts of adults living with LOPD treated with cipaglucosidase alfa plus miglustat that are different from those studied in clinical trials (eg, transitioning from avalglucosidase alfa)
- Genotype-phenotype link
- Enhance understanding of the relationship between genotype and clinical phenotype, including severity, disease progression and treatment response
- Timely intervention
- Evaluate the impact of timely intervention (including switch) on clinical outcomes and long-term disease trajectory
- Improving time to accurate diagnosis
- Biomarkers
- Identification of novel biomarkers useful for prognosis and assessment of treatment response
- Translational research to understand the relationship between existing biomarkers and long-term clinical outcomes e.g. CK and HEX4
- Mechanism of action
- Translational research to further characterize the mechanism of enzyme delivery and activity for available therapies on pathobiology, pathophysiology and clinical outcomes
- Clinical management
- Characterize the criteria (e.g. clinical, patient and biomarkers) for transitioning from one available therapy to another and assess outcomes
- Use of advanced technology to support patient monitoring e.g. wearables
*Amicus reserves the right to modify, revise or delete the Areas of Interest and other terms and conditions of its IIS program at any time without notice.
IIS Review Process:
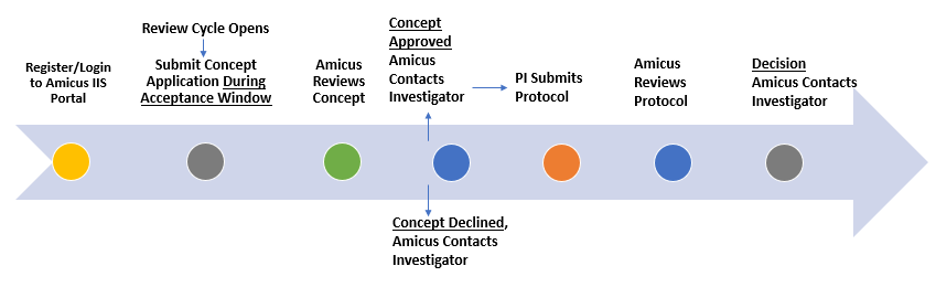
Concept Application
The IIS review process begins with submission of a study concept. The concept document and a copy of your signed and dated CV must be uploaded onto the IIS portal.
Amicus strives to provide a response to your concept application within 30 days of the scheduled review date.
Acceptance or approval of the concept does not guarantee support of a study.
Protocol Application
If your IIS concept application has been approved, you will be notified of the decision and can begin drafting the study protocol. To facilitate this process and ensure all necessary information is reflected in the protocol, we provide a template that can be found on the Welcome Page of the IIS portal.
Submit your research protocol, budget (template provided), and other supporting documents.
New Users REGISTER on IIS Portal – to begin the Concept Application submission process.
Returning Users GO to IIS PORTAL – to track the status of your application.
Amicus strives to provide a response to your protocol application within 30 days of the scheduled review date.
Note: A proposal requesting Amicus support (e.g., funding and/or drug supply) is not a guarantee of acceptance or approval of that proposal. Amicus support will only be provided upon the execution of an agreement.
Thank you for your interest in the Amicus Therapeutics, Global Medical Affairs Investigator-Initiated Studies. Please email the Investigator Initiated Studies Administrator at Investigator-InitiatedProgram@amicusrx.com with additional questions.
MED-ALL-NN-2400004